Dental Fluorosis
The First Warning Sign of Toxicity
In our last issue, The Truth About Fluoride, we traced how fluoride became embedded in public health policy. We learned that its addition to public water and toothpaste was never based on fulfilling a biological need - there was no evidence the human body needed it. Instead, industry influence shaped its adoption, rebranding chemical waste as a “public health” solution.
That history matters because it reframes how we view fluoride today. Unlike minerals the body truly needs—such as calcium, magnesium, and phosphorus—fluoride is not essential: it does not strengthen teeth, it does not build healthy bones, and it isn’t required by any organ system. Even major health organizations like the FDA, CDC, and WHO now acknowledge that fluoride provides no essential health benefit.
This raises an important question: if the body has no use for fluoride, what happens when we ingest it? In this issue, we’ll cover how fluoride is absorbed and stored in your body, why it builds up in teeth and bones, and how this quiet accumulation can lead to irreversible health consequences.
Contents
Fluoride in the Body
Why Fluoride Accumulates in Bones and Teeth
What Is Dental Fluorosis?
Stages of Dental Fluorosis
Health Effects Beyond Tooth Enamel
Protect Your Family
Absorption
When you ingest fluoride – whether from drinking water, toothpaste, or foods – your body absorbs most of it. Approximately 80–90% of ingested fluoride is rapidly absorbed through the gastrointestinal tract into the bloodstream.
Once absorbed, fluoride disperses throughout the body via the blood circulation. Unlike nutrients whose levels are tightly regulated by the body, fluoride is not subject to any homeostatic control in our blood. It goes wherever the blood flows, chemically reacting with organs and tissues as it circulates.
Retention and Accumulation
After absorption, the kidneys work to filter toxins out of the blood, but their capacity is limited when it comes to fluoride. On average, only about 50% of the fluoride an adult ingests each day is excreted in urine; the other half remains in the body, where it is free to roam and gradually accumulates as a cumulative toxin.
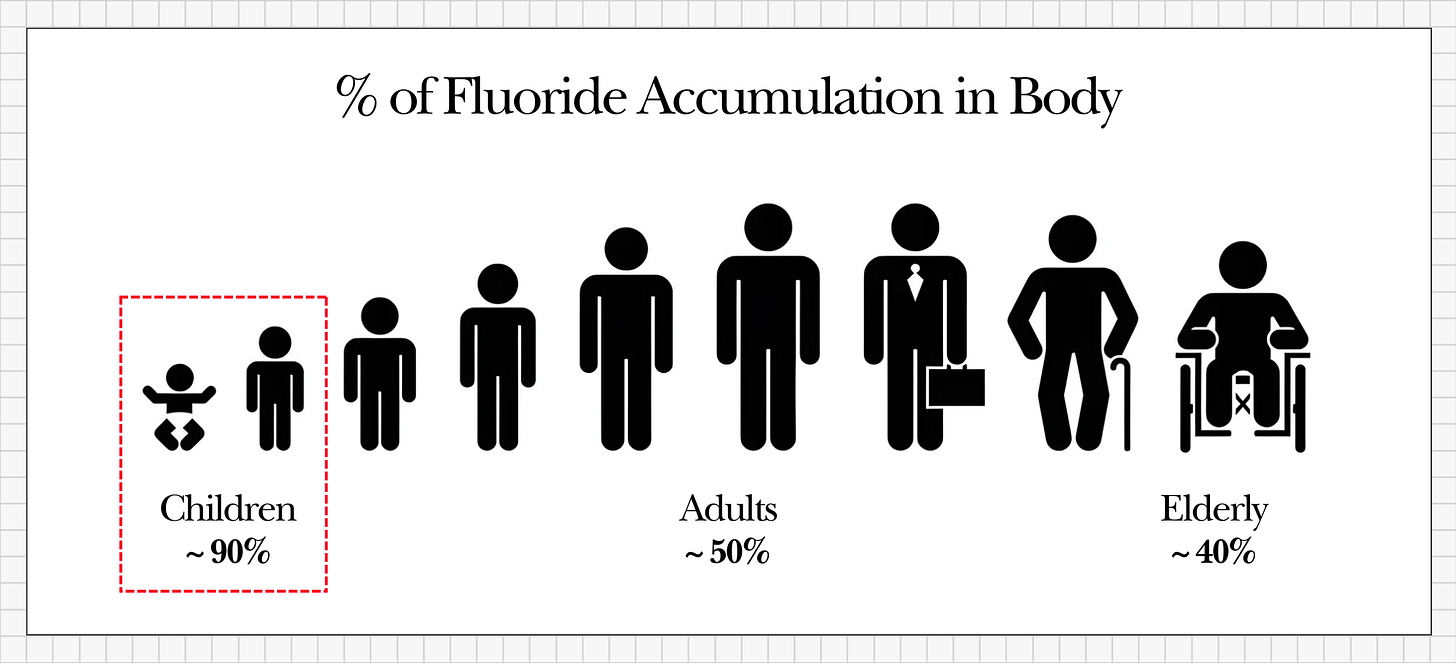
This retention rate is even higher in the very young. Children may retain up to 60–80% of absorbed fluoride, and infants under one year old can retain as much as 90%.
What makes fluoride’s accumulation especially concerning is that the human body has no efficient mechanism to remove stored fluoride. Once fluoride lodges in our tissues, there is no enzyme or metabolic pathway that can quickly dislodge it. Only very slow processes - such as the turnover of bone tissue or the tiny amount of fluoride released from bone and excreted by the kidneys - can reduce the body’s fluoride burden, and these processes occur over years.
For example, even after stopping fluoride intake completely, studies have found that bone fluoride levels might fall by only about 50% after 20 years. In practical terms, if you stopped ingesting fluoride today, it would take two decades for your body to eliminate just half of what has already accumulated. Each new exposure simply adds to the cumulative burden. Layer by layer, year after year, fluoride quietly embeds itself deeper into our tissues.
Why Fluoride Accumulates in Bones and Teeth
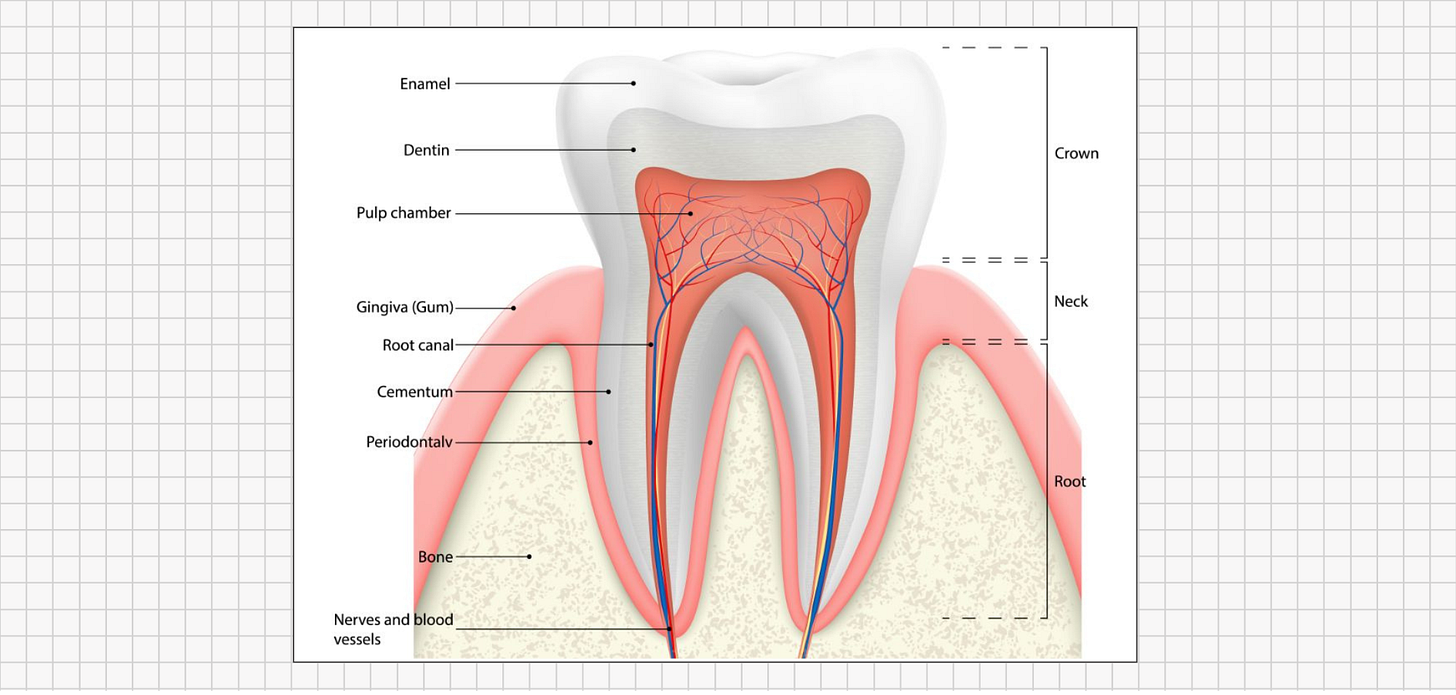
When fluoride enters the bloodstream, it doesn’t distribute evenly across all organs; instead, it gravitates toward calcium-rich tissues. Roughly 99% of the fluoride that remains in the body is ultimately stored in bones and teeth.
This is no coincidence of anatomy but rather a predictable result of chemistry. Fluorine (the elemental form of fluoride) is the most electronegative element on the periodic table, which means it strongly attracts positively charged minerals like calcium. In effect, fluoride seeks out areas where calcium is abundant and binds tightly to those calcium-rich mineral matrices. This chemistry explains why our teeth and bones become the primary storage sites for fluoride.
This selective deposition sets the stage for the earliest visible sign of excess fluoride exposure during development: dental fluorosis.
Dental fluorosis is a developmental disturbance of tooth enamel caused by excess fluoride exposure during the years when teeth are forming. It isn’t a genetic disease or a random defect; it’s a visible outcome of too much fluoride in a child’s body during a critical growth period. In essence, dental fluorosis is the evidence, written on your teeth, that fluoride disrupted the normal enamel-building process.
Timing Matters: The “Critical Window”
Teeth form long before they erupt into the mouth, and enamel is built only once. There are specific “critical windows” in early life when excessive fluoride can damage developing teeth:
Baby (primary) teeth: Begin forming around the 14th week of pregnancy and finish mineralizing by about age 3.
Permanent (adult) teeth: Start forming at birth and continue developing under the gums until roughly age 8 (wisdom teeth extend into the teenage years).
If fluoride intake is high during these developmental windows - whether from water, toothpaste, mouth rinses, or other sources - the normal enamel-forming process is disrupted. When fluorosis later appears on the teeth, it signals that a child’s fluoride exposure reached toxic levels at some point during enamel development.
How Healthy Enamel Forms
To appreciate what goes wrong in fluorosis, it helps to first understand how healthy enamel is formed.
Enamel formation is a precisely orchestrated biological process. Specialized cells called ameloblasts secrete and organize the enamel tissue in stages. Initially, ameloblasts produce a protein-rich matrix as a scaffold. Then, during the enamel maturation stage, those proteins are gradually removed and replaced with tightly packed crystals of hydroxyapatite (a calcium-phosphate mineral). The result under ideal conditions is a dense, glass-like lattice of mineral; mature enamel is about 96% mineral, making it the hardest tissue in the human body. Healthy enamel comes out extremely smooth, hard, and resistant to wear - essentially a suit of armor designed to last a lifetime.
How Excess Fluoride Disrupts Enamel Formation
When excess fluoride is present during those critical periods of enamel formation, it derails the maturation process. Fluoride can interfere with the removal of proteins and the orderly deposition of minerals. At the atomic level, fluoride ions swap in for the normal hydroxyl groups in the hydroxyapatite crystal lattice. This seemingly small substitution has a big impact: instead of growing smooth, tightly packed enamel crystals, the presence of fluoride distorts the crystal growth. The developing enamel ends up with microscopic gaps and irregularities. In short, too much fluoride makes enamel that is more porous, less organized, and permanently weaker.
Stages of Dental Fluorosis
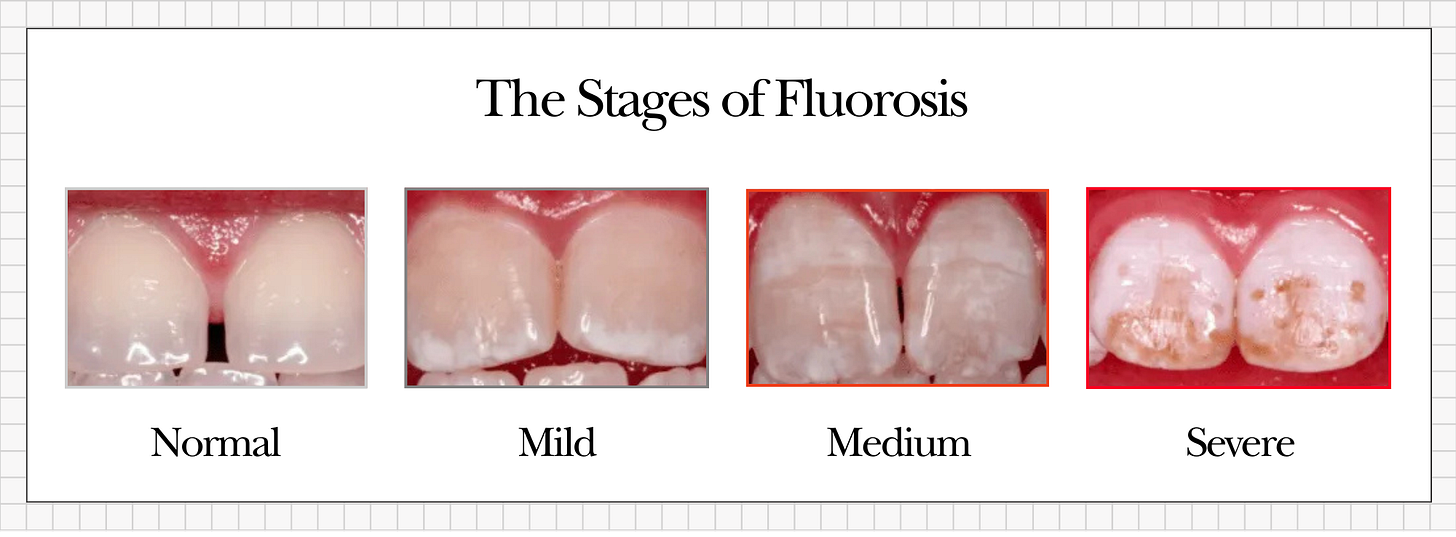
The severity of dental fluorosis depends on how much fluoride a child consumed (and for how long) during tooth development. When the affected teeth finally erupt into the mouth, the damage becomes visible. Dentists generally categorize fluorosis severity as follows:
Mild fluorosis: Faint white streaks or tiny opaque specks on the enamel surface.
Moderate fluorosis: Larger chalky white patches on enamel, often with a rougher texture.
Severe fluorosis: Distinct brown stains, significant pitting of the enamel, and areas so brittle that the enamel can chip or flake away.
Mild dental fluorosis appears as faint white streaks or specks on tooth enamel. In the photo above, you can see small opaque white patches on the upper front teeth – this is characteristic of a mild case. These subtle markings might be easy to overlook or dismiss as purely “cosmetic,” but they are an early red flag that excessive fluoride disrupted the enamel’s formation.
Severe dental fluorosis results in brown stains, pitted surfaces, and fragile enamel. The image above shows a severe case: the teeth exhibit deep brown discoloration and a corroded, pitted appearance. In cases this advanced, the enamel’s structure is so compromised that pieces of it may break off. This level of damage is impossible to ignore and starkly illustrates the toxic effects of fluoride overexposure during development.Enamel affected by fluorosis is permanently altered and structurally weaker than normal enamel. It is more porous, which makes it more prone to chipping, wearing down, and decay. Its increased porosity also means it stains more readily.
Public health officials often dismiss mild fluorosis as “only cosmetic,” but that is a mischaracterization. Dental fluorosis is a visible warning sign of toxicity. Even the mildest white flecks indicate that fluoride reached toxic levels in the body during a crucial period of tooth development. In severe cases, the damage speaks for itself.
Health Effects Beyond Tooth Enamel
Dental fluorosis is only the beginning; it marks the first visible evidence of broader health risks from fluoride. While the most immediate effect of fluoride toxicity appears in the teeth, continued exposure and accumulation can lead to a host of other issues throughout the body.
Extensive research, including animal studies, human epidemiological findings, and clinical reports, has linked long-term fluoride ingestion to problems in multiple organ systems. Here are a few documented conditions associated with chronic fluoride exposure:
Skeletal fluorosis: Fluoride gradually builds up in bone, making bones abnormally dense but paradoxically more brittle. Over time this can cause joint stiffness, arthritis-like pain, and an increased risk of fractures.
Neurological effects: High fluoride exposure has been linked in some studies to reduced IQ in children, and associations have been observed with behavioral issues such as ADHD and certain autism spectrum disorder symptoms.
Thyroid disruption: Fluoride can compete with iodine in the body and may suppress normal thyroid hormone production. This can contribute to hypothyroid-like effects – for example, fatigue, weight gain, and in children, developmental delays.
These examples represent only part of fluoride’s impact. Given enough time and continued exposure, fluoride has the potential to affect virtually every system it reaches in the body. From the brain to the bones, no tissue is truly immune to fluoride’s effects if the exposure is high and long-lasting.
Final Thoughts
Fluoride has long been lauded as a “public health success,” but the evidence tells a different story. Dental fluorosis shows us, in plain sight, that excess fluoride can disrupt one of the body’s most precise developmental processes. Our hardest, most resilient tissue – tooth enamel – becomes porous, stained, and fragile under fluoride’s influence. And if fluoride can so profoundly alter enamel formation, it’s not hard to imagine the subtler, less visible effects it might be having on our bones, glands, or even our brains.
Public health agencies may downplay mild fluorosis as a purely cosmetic issue, but those faint white streaks are not harmless blemishes. They are early warning signs – markers that the body has been overloaded with a substance it neither needs nor can efficiently eliminate. Fluorosis isn’t just about spotting discolorations on teeth; it’s a window into fluoride’s systemic impact on our health.
The key takeaway is simple: fluoride is not essential to any biological process, yet it accumulates in our bodies over time. By the time dental fluorosis spots appear on teeth, fluoride has already been building up internally and leaving its mark elsewhere. Recognizing this reality is the first step in protecting ourselves and our families from unnecessary harm.
Protect Your Family
The good news is that dental fluorosis is entirely preventable. If you or your child already show signs of fluorosis, take it seriously - it’s an early warning that fluoride has begun accumulating in the body. If no signs are visible, consider that good news and a window of opportunity to keep fluoride exposure low.
In either case, the solution is the same: reduce and eliminate unnecessary fluoride exposures from your daily life. Here are some concrete steps you can take:
Water: Use a high-quality reverse osmosis (RO) or distillation system (with third-party testing) to remove fluoride from your tap water. (ideally one with third-party testing to confirm fluoride removal). Most simple pitcher or faucet filters do not eliminate fluoride. If you rely on bottled water, look for brands labeled “fluoride-free,” or check the water quality report to ensure low fluoride levels.
Toothpaste: Switch to fluoride-free toothpaste, especially for young children. Kids under six often swallow toothpaste, and even a pea-sized dab of standard 1000–1500 ppm fluoride paste is a significant dose if ingested twice daily. Also, avoid fun flavors or sparkly toothpastes that might encourage children to swallow their toothpaste.
Nutrition: Ensure your diet includes nutrients that can help counteract fluoride. Sufficient calcium and magnesium, for example, can compete with fluoride in bones and teeth, reducing fluoride’s binding and absorption. A nutrient-dense diet with whole foods supports overall tooth and bone health. Foods rich in fat-soluble vitamins (such as vitamin D and K2 from egg yolks, grass-fed butter, bone broth and organ meats) also help strengthen enamel and bone, making them more resilient to toxins.
Consistency: Focus on eliminating fluoride from the two biggest sources in most households: your drinking water and your toothpaste. These changes have the largest impact. Once you cut off those major sources – and stay consistent about it – your body can gradually start to clear out some of the fluoride that has accumulated over time.
By taking these steps and remaining vigilant, you can significantly reduce new fluoride exposure and prevent further accumulation in your body. Each action you take limits how much additional fluoride you ingest, giving your body a chance to slowly detoxify and recover from the fluoride that’s already been stored.
Coming Next
In our next issue, we’ll take a closer look at skeletal fluorosis — examining how long-term fluoride exposure changes bone chemistry, stiffens joints, and erodes mobility. From there, we will continue tracing fluoride’s effects throughout the body in this series.
This ongoing series is designed to give you the full picture of what fluoride does to the human body, why that damage matters, and most importantly, how you can protect yourself and your family. Stay tuned!